Covid-19 mouse models available at CCP
Ace2 knock-out model
A number of promising COVID-19 therapies is focused on targeting the Ace2 receptor. Thus, it is desired to evaluate:
- the potential of Ace2 blocking to prevent viral entry into the cells
- unwanted off-target effects caused by Ace2 deficiency. Ace2 deficient mutant mice provide an excellent tool for these types of experiments.
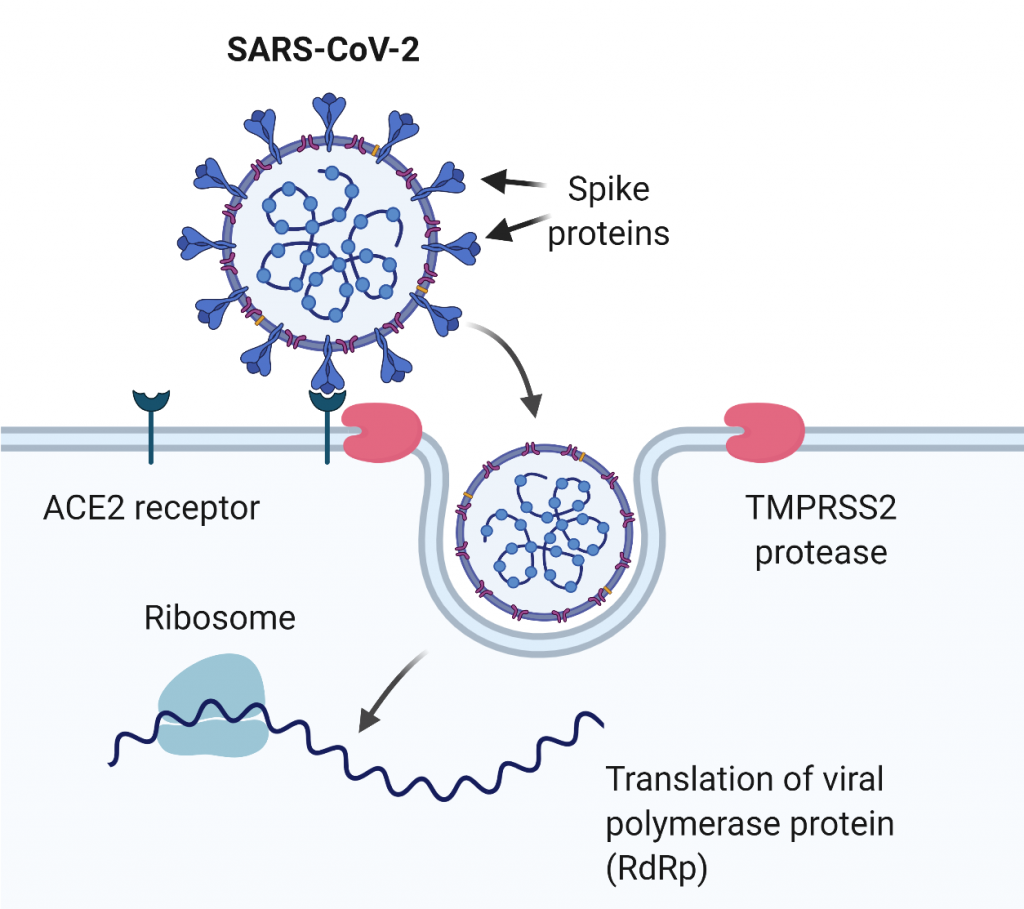
Tmprss2 knock-out model
mPRSS2 ablation was previously shown to partially rescue severe lethal phenotype upon SARS-CoV infection as it prevents efficient viral replication and dissemination, and leads to less severe immunopathology compared to the wild type mice. TMPRSS2 KO mice can be used in the research of COVID-19 infections to investigate the pathophysiological role of TMPRSS2 and to validate TMPRSS2 as a potential therapeutic target.
Humanized hAce2 models
Human and mouse ACE2 enzymes share about 80% homology and similar expression pattern. However, the mouse Ace2 variant differs in the viral receptor-binding domain, therefore SARS-CoV and SARS-CoV2 infections are mediated with a dramatically lower efficacy. Consequently, wild type mice are highly resistant to these viruses and their use in SARS or COVID-19 research is limited. An obvious strategy to circumvent this problem was the generation of mouse models carrying human Ace2 variants in their genome.
- Conditionally overexpressing hAce2 KI (Rosa26)
Will allow tissue-specific overexpression upon the breeding of the chAce2 line with a specific CRE line. Mice were generated by insertion of the construct carrying CAG promoter, a STOP cassette flanked by loxP, followed by hAce2 cDNA. The targeting cassette was inserted into Rosa26 safe harbour locus. - Endogenous Ace2 KI (mAce2 locus)
Will allow hAce2 expression driven by endogenous Ace2 promoter. Mice were generated by insertion of hAce2 cDNA into the second coding exon of mAce2, keeping the endogenous regulatory sequences intact. N-terminal part of the mAce2 protein is separated from the human variant by the p2a sequence.