Preclinical Testing
Customized attitude to your project – services from routine measurements to study design & run packages
• Reference values – range of values typical for healthy animals (C57Bl/6N mice) (from CCP and IMPC phenotyping, standard for interpretation of results)
• GLP/non GLP mode
• Expertise in mouse adult AND prenatal development
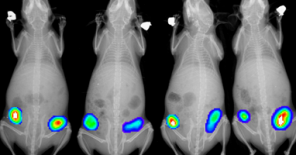
• Advanced diagnostic capabilities for any drug testing or research in vivo imaging (many modalities, from cells to whole body), various functional tests of mouse/rat physiology, metabolism, senses and behavior, immunology, histopathology, clinical biochemistry and hematology
• Advanced surgery skills – intra-organ application of cells or drugs (ovary, mammary gland, pancreas, kidney, liver etc.)
• Advanced usage of cell cultures and molecular biology tools cell cultures and targeted gene modifications (CRISPR/Cas9 technology, other systems – AAV, AV, lentiviral vectors etc.), organoids
• Advanced animal models prepared in house – genetically modified or specifically induced
More info
We can prepare and proceed projects from a broad variety of physiological categories from single parameter analysis (for example glucose level) to multi parametric analysis using panels from clinical biochemistry, immunology, cytokine levels etc. The preclinical pipeline can be also tailored from more physiological assesments across the whole CCP portfolio.
| Analysis of samples | Other preclinical testing |
| – Hematological and biochemical examination of samples – Histopathological evaluation of tissues – Immunoadsorption methods (ELISA, Luminex) – Flow cytometry – Urineanalysis – LC/MS analysis – body liquids, cells, tissues | – qAcute or chronic toxicity of tested items on mice/rats – qPharmacokinetics and bio-distribution in vivo – mice or rats – qVarious disease/pathology mouse models – aimed to pharmacodynamics/efficacy drug testing – qStudies on infectious diseases – BSL3 safety level available for animal experiments |
For requests on preclinical tests/other studies directed to a treatment development, please complete the “Request services” form. For general inquiries, please complete the contact form below.
Coordinator of preclinical studies: Gizela Koubkova